Provided the bit is comfortable there should now be nothing that interests the horse about the bit that can become a distraction to the rider’s aids. This includes tastes and smells which are the remaining sensory functions relevant in an object intended for the mouth.
It is known that divalent metal oxides, particularly of copper and iron can excite such sensory responses. Groundbreaking research in 20061 showed that these divalent metal ions in contact with epithelial cells stimulate the production of volatile carbonyl hydrocarbons which account for the odour of metals and odour is an essential part of the experience of taste. These volatile organic compounds are essentially ‘body odours’, but whether they are favourable or unfavourable to the horse they may produce an over-activity in the mouth which is unwanted.
Stainless steel is the perfect example to illustrate how such stimulants can be suppressed. The insoluble protective layer of chromium dioxide on the surface of stainless steel locks in alloy metals such as iron and nickel whose oxides would otherwise become bio-available. This also explains why no nickel allergy is expected from stainless steel.
Following this idea, Neue Schule adds aluminium to the mouthpiece metal composition. It is known that aluminium does not produce the volatile compounds but it produces a protective oxide (alumina, Al2O3) that in part prevents the bit surface from becoming rich in oxides of copper and zinc. A study comparing Salox with an aluminium-free SCA (with closest equivalent standard composition, UNS C69400) strikingly emphasises the relative reduction in extractable copper and zinc due to this effect.
Thin discs (12.5 mm diameter, 1 mm thickness) were cut from a Neue Schule Salox bit and from an alternative standard copper alloy mouthpiece. The discs were first solvent cleaned and pre-treated in oxygen plasma to ensure full oxidation of the surface. Aqueous extracts were measured by ICP emission spectroscopy after 6 days reflux into boiling phosphate buffer solution at pH7.
Based on the known relative quantities of copper and zinc between the two samples and making no other assumptions the expected extraction quantities should be proportional to the metal content (‘expected’ data). The measured data, shown in figure 2, show a 4 fold relative reduction in zinc and a 2 fold relative reduction in copper as soluble extracts compared to the expected values.
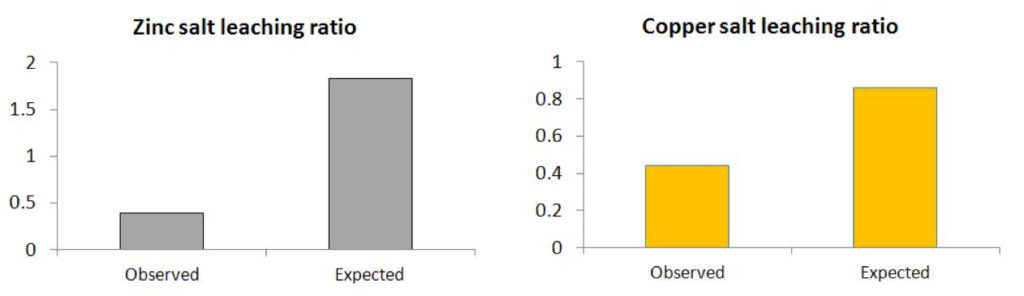
Figure 2: Observed ratio (Salox : SCA) of extracted copper and zinc compared with Expected ratio based on weight percent composition ratio.
Aluminium clearly offers the same method of protection as chromium in stainless steel and gets closer to our goal of inhibiting the availability of copper and zinc ions in contact with the mouth. This will reduce the production of the organic compounds responsible for taste and smell.